Pathogen and Medication Partner Up to Fight Polymicrobial Infections
Polymicrobial diseases, which are recognized with increasing frequency, are acute and chronic diseases caused by various combinations of viruses, bacteria, fungi, and parasites. Generally the presence of one micro-organism generates a niche for other pathogenic micro-organisms to colonize, the presence of one microorganism predisposes the host to colonization by other microorganisms, or two or more non-pathogenic micro-organisms together cause disease. Polymicrobial infections also include an interaction called microbial interference: the presence of one micro-organism generates a niche in the host that suppresses the colonization of other microorganisms. Microbial interference can occur between potential pathogens or between probiotic organisms and pathogens.
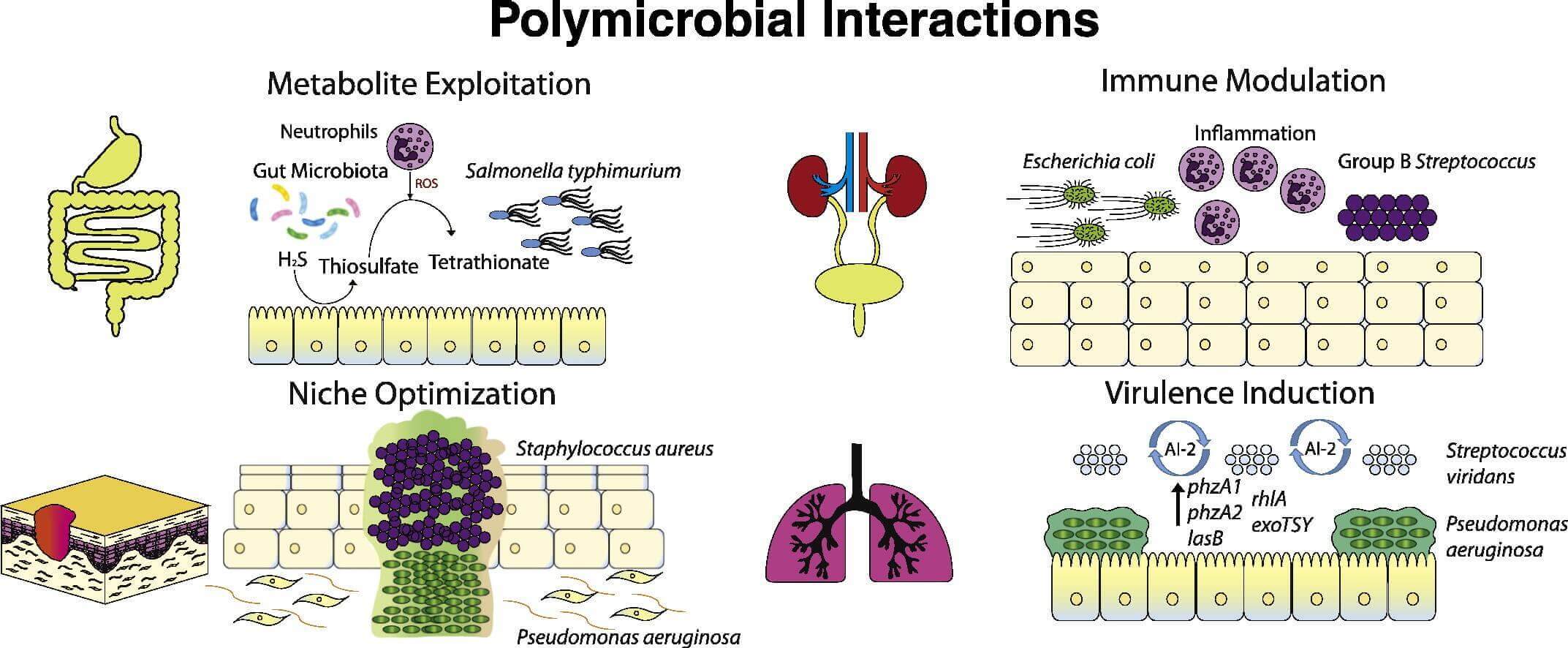
Human metapneumovirus, for example, has been isolated with coronavirus in patients with severe acute respiratory syndrome and also with respiratory syncytial virus in bronchiolitis and other respiratory infections. Measles virus kills mainly because of secondary infections induced by immunosuppression, and an immunosuppressive factor produced by infected lymphoid cells might profoundly inhibit B-cell proliferation. Many HIV-AIDS patients in Africa are also co-infected with malaria parasites or with Mycobacterium tuberculosis, and with other viruses, bacteria, fungi, and protozoans. The role of Epstein-Barr virus and retrovirus resulting in multiple sclerosis is still to be determined.
Polymicrobial infections are challenging to treat because scientists don’t fully understand how pathogens interact during infection and how these interactions impact the drugs used to treat them. Traditional therapies are generally targeted at individual causative agents without consideration for effect on a polymicrobial cause or on individual members of microbial communities.
In a study published in the journal Infection and Immunity, researchers in the Molecular & Biomedical Sciences Department looked at two pathogens that often occur at similar sites, particularly in cystic fibrosis and mechanically ventilated patients: Candida albicans and Pseudomonas aeruginosa. Opportunistic microbes co-inhabit diverse host niches, leading to difficult-to-treat co-infections of immunocompromised individuals. However, we know little about how host tissue and microbe-microbe interactions affect antimicrobial sensitivity.
Candida albicans and Pseudomonas aeruginosa are two of the most prolific opportunistic pathogens in the developed world, inhabit the same sites and are associated with polymicrobial infections. Candida is the fourth most common nosocomial pathogen and Pseudomonas is also associated with significant mono-microbial disease. Candida-Pseudomonas co-infections are associated with exacerbated disease, but it is not clear if co-infection should be treated with the same antimicrobials as mono-infection.
Previous studies have predominantly focused on physical and molecular interactions between C. albicans and P. aeruginosa and their effect on growth, morphology and virulence, but little is known about effects of cohabitation with antimicrobial treatment. Fluconazole tolerance is high among some clinical isolates and is associated with empirical treatment failure and worse outcomes.
To investigate if C. albicans-P. aeruginosa interactions affect fluconazole efficacy, the researchers studied its activity in vitro and in the zebrafish infection model. To determine if interactions between these microbes affect antimicrobial sensitivity, the researchers performed coculture experiments to test if P. aeruginosa affects the antifungal action of fluconazole (FLC) against C. albicans. While FLC is fungistatic in vitro against C. albicans, in C. albicans - P. aeruginosa coculture it has potent fungicidal activity. FLC alone slowed growth of C. albicans while P. aeruginosa alone showed little to no effect on C. albicans growth, however the combination led to killing of greater than 1000x from the initial fungal inoculum. This loss of fungal viability is a hallmark for loss of FLC tolerance.
To further examine C. albicans-P. aeruginosa interactions in the presence of FLC in vivo, the researchers leveraged their zebrafish swimbladder co-infection model. They found that FLC treatment significantly reduced mortality in co-infection, although there was only a trend toward reduced mortality in the mono-infected group. This indicates that FLC is more effective in treating C. albicans-P. aeruginosa co-infection than fungal mono-infection, suggesting that there is also bacterial-drug synergy in vivo.
What the study found was promising. The results showed that P. aeruginosa works with fluconazole to eliminate drug tolerance and clear the C. albicans infection in the culture and the zebrafish. “Polymicrobial infections are challenging to treat not only because of the lack of understanding of how invading microorganisms interact but also because we don’t know how these interactions affect treatment efficacy. Our work demonstrates that polymicrobial interactions can indeed affect treatment efficacy and, most importantly, it highlights the importance of nutrient availability in the environment — such as iron in our study — and how it modulates treatment efficacy,” says Siham Hattab, lead author of the study who conducted the research as part of her Ph.D. in the Department of Molecular and Biomolecular Sciences.
This study also implicates iron in infection and therapy in a new way beyond strictly as a micronutrient subjected to sequestration by the host and pathogen. Iron is an important micronutrient for both C. albicans and P. aeruginosa, and iron chelation leads to enhanced FLC activity against C. albicans. The researchers supplemented cocultures of C. albicans and P. aeruginosa and FLC with 1 mM FeCl3 in vitro. It was found that iron supplementation limits but does not eliminate the synergistic fungicidal activity of the P. aeruginosa-FLC combination. The clear loss in fungal viability even upon iron supplementation is consistent with an inability for iron to restore FLC tolerance. Nonetheless, clinical studies of iron chelation against fungal infection are inconclusive and suggest it may negatively impact health. Thus, the prospect of using iron chelation to increase drug effectiveness during treatment of human patients holds both risks and potential benefits.
“We are really excited to have revealed that sometimes drugs against fungal infection can work even better in a more ‘real-world’ situation than in the test tube. There is still a lot to learn about how pathogens interact during infection, and it will be interesting to see how the bacteria manage to work with the drugs to target Candida,” says Robert Wheeler, associate professor of microbiology and senior author of the study. On a more general level, our results also suggest that the biotic and abiotic growth environment can influence the efficacy of antifungal drugs, pointing the way toward new strategies for developing drugs to eradicate recalcitrant infections.
About Fluconazole
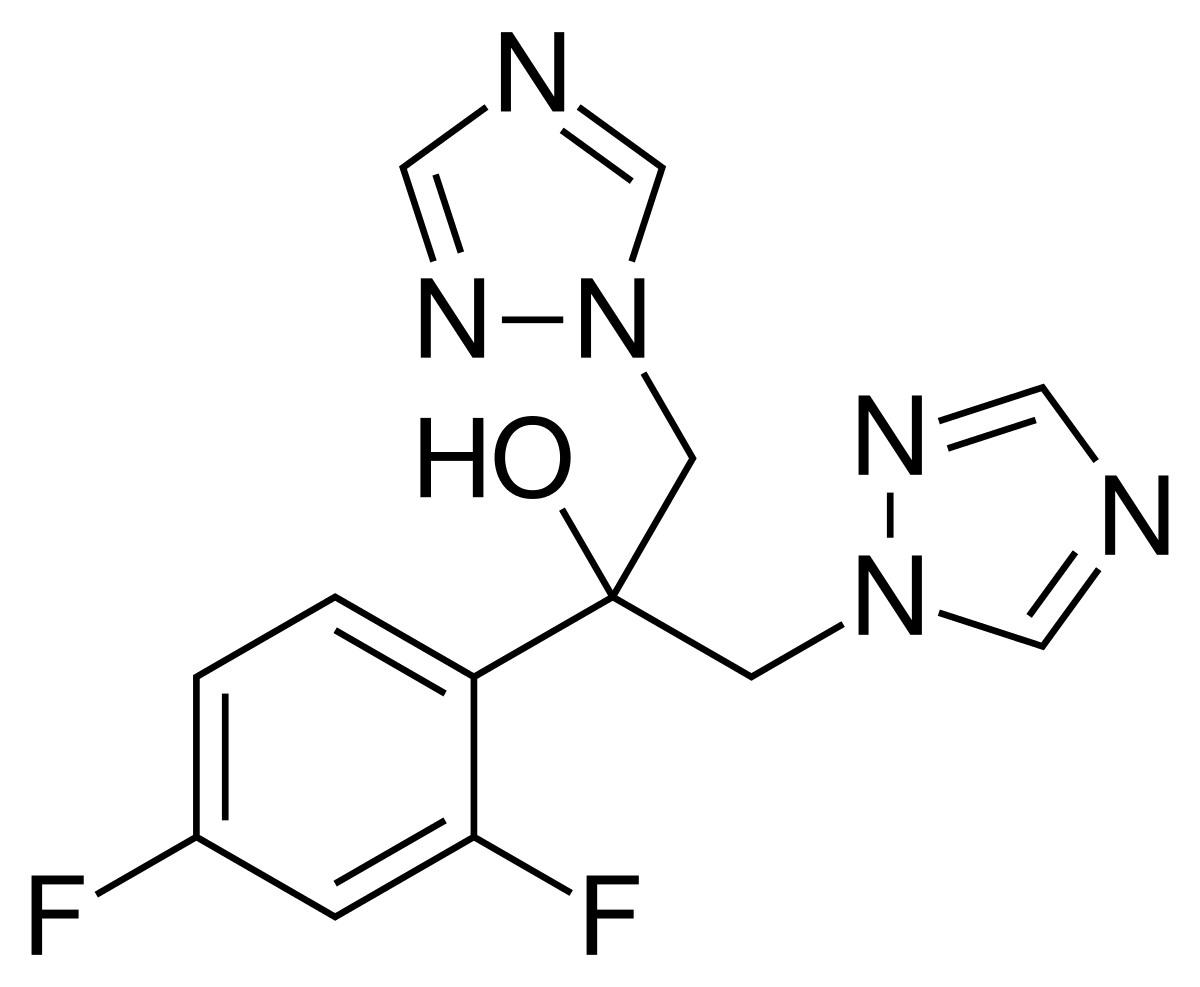
Fluconazole, commonly known as Diflucan, is an antifungal drug used for the treatment of both systemic and superficial fungal infections in a variety of tissues. Fluconazole is a very selective inhibitor of fungal cytochrome P450 dependent enzyme lanosterol 14-α-demethylase. This enzyme normally works to convert lanosterol to ergosterol, which is necessary for fungal cell wall synthesis. The free nitrogen atom located on the azole ring of fluconazole binds with a single iron atom located in the heme group of lanosterol 14-α-demethylase. This prevents oxygen activation and, as a result, inhibits the demethylation of lanosterol, halting the process of ergosterol biosynthesis. Methylated sterols are then found to accumulate in the fungal cellular membrane, leading to an arrest of fungal growth. These accumulated sterols negatively affect the structure and function of the fungal cell plasma membrane.
Fluconazole was patented in 1981 and came into commercial use in 1988. It is on the World Health Organization's List of Essential Medicines. Fluconazole is available as a generic medication. In 2019, it was the 133rd most commonly prescribed medication in the United States, with more than 5 million prescriptions.
Other inhibitors and antagonist of Lanosterol 14-α-demethylase
- Miconazole
- Oxiconazole
- Bifonazole
- Sertaconazole
- Clotrimazole
- Econazole
- Posaconazole
- Terconazole
- Tioconazole
- Voriconazole
- Luliconazole
References
- Hattab, Siham, Anna-Maria Dagher, and Robert T. Wheeler. "Pseudomonas synergizes with fluconazole against Candida during treatment of polymicrobial infection." bioRxiv (2021).