Investigating Properties of Cow Milk Protein Lactoferrin Against SARS-CoV-2 Variants
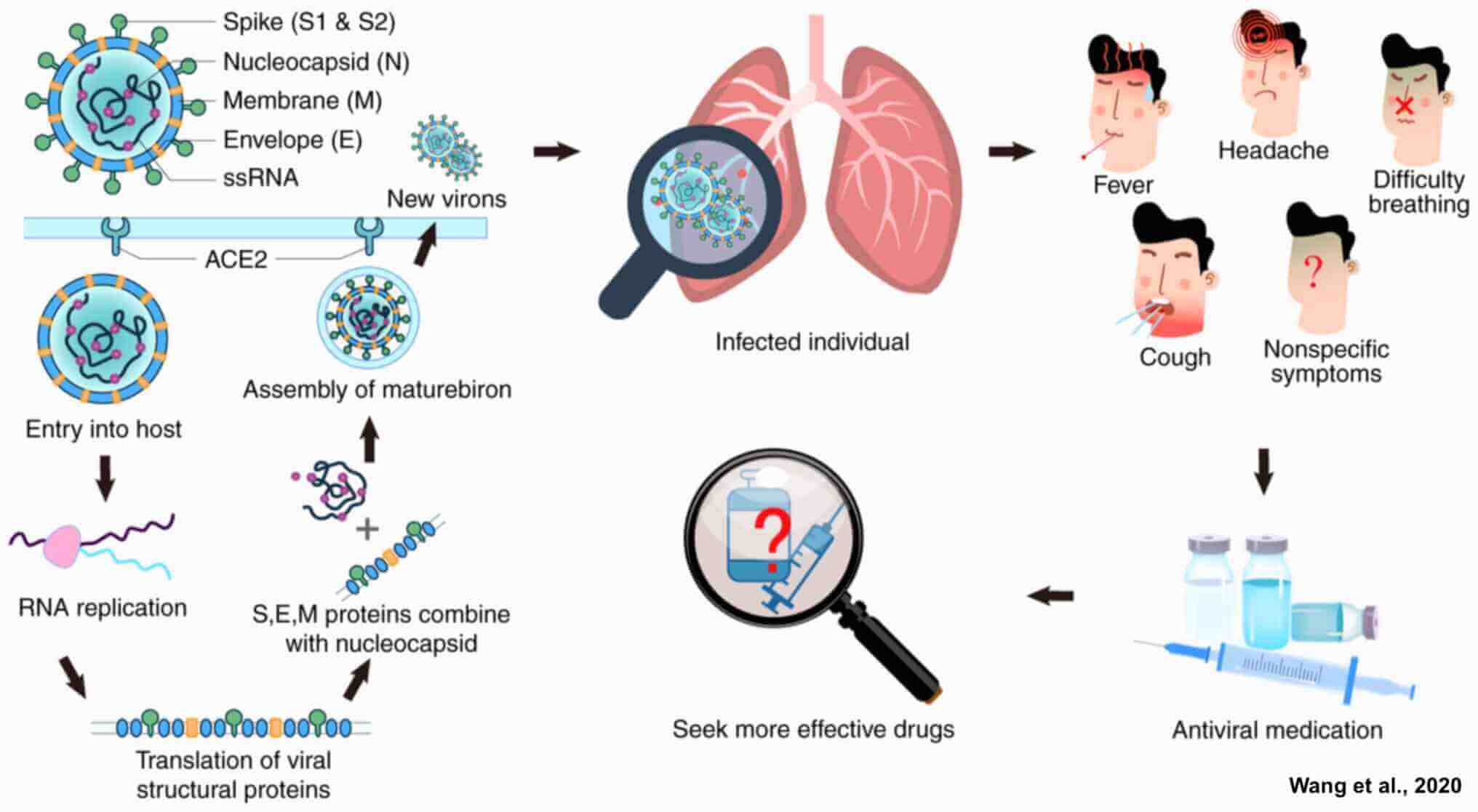
As we enter the third year of the pandemic, SARS-CoV-2, the virus that causes COVID-19, is spreading between people at an intense level globally. SARS-CoV-2 is a member of a large family of viruses called coronaviruses. These viruses can infect people and some animals. SARS-CoV-2 was first known to infect people in 2019. Like other coronaviruses, SARS-CoV-2 has four structural proteins, known as the S (spike), E (envelope), M (membrane), and N (nucleocapsid) proteins; the N protein holds the RNA genome, and the S, E, and M proteins together create the viral envelope. Coronavirus S proteins are glycoproteins and also type I membrane proteins (membranes containing a single transmembrane domain oriented on the extracellular side). They are divided into two functional parts (S1 and S2). In SARS-CoV-2, the spike protein, which has been imaged at the atomic level using cryogenic electron microscopy, is the protein responsible for allowing the virus to attach to and fuse with the membrane of a host cell; specifically, its S1 subunit catalyzes attachment, the S2 subunit fusion. The virus is thought to spread from person to person through droplets released when an infected person coughs, sneezes, or talks. Very few drugs are known to effectively inhibit SARS‑CoV‑2. Unfortunately, according to the Organization for Economic Co-operation and Development, much of the developing world will not reach mass immunization until 2024, leaving a large population of people vulnerable to the disease.
As the COVID-19 pandemic continues to claim lives around the world, dairy scientists may have a surprising role to play. In a new report published in the Journal of Dairy Science, scientists from the University of Michigan and Glanbia PLC Research and Development have collaborated to investigate the antiviral properties of cow milk protein against variants of SARS-CoV-2.
Lactoferrin (formerly known as lactotransferrin) is a glycoprotein, and a member of a transferrin family, thus belonging to those proteins capable of binding and transferring Fe3+ ions. The predominant cell types involved in lactoferrin synthesis are of the myeloid series and secretory epithelia. In adults, higher levels of lactoferrin are present in milk and colostrum. It is found in the milk of most mammals and is part of the innate immune system. Lactoferrin displays a wide variety of bioactive characteristics endogenously as part of the innate immune system. Due to the increase in its concentration during most inflammatory reactions and some viral infections, several authors classify lactoferrin as an acute-phase protein. Based on previous studies, bovine lactoferrin (bLF) displays broad-spectrum antiviral activity both in vitro and in vivo. In vitro results show that bLF inhibits respiratory syncytial virus (RSV), influenza A virus (H3N2, H1N1, H3N2), as well as avian influenza A virus (H5N1), rotavirus, adenovirus, poliovirus, echovirus, herpes simplex virus (HSV-1, HSV-2), and other viruses. Bovine lactoferrin has also shown antiviral activity in human clinical trials. For example, orally administered bLF has been shown to improve the severity of viral infections including rotavirus and norovirus. Because of this, several review papers have suggested using bLF as a prophylactic or postexposure treatment for SARS-CoV-2 infection.
With the goal of improving clinical relevance and translatability, the team tested bovine lactoferrin against some of the most common SARS-CoV-2 variants of concern from around the world, including the WA1 variant representative of the United States outbreak in 2020, the B.1.1.7, B.1.351, and P.1 variants, and the Delta variant. A previous study from the same group in 2021 has reported that, from a library of 1,425 US FDA-approved compounds and clinical candidates, we identified 17 hits that inhibited SARS-CoV-2 infection and analyzed their antiviral activity across multiple cell lines. Among all the hits, they discovered that lactoferrin, a glycoprotein found in secretory fluids including mammalian milk, inhibits SARS-CoV-2 infection in the nanomolar range in all cell models with multiple modes of action, including blockage of virus attachment to cellular heparan sulfate and enhancement of interferon responses.
The team's aims for this study were to expand upon the observation of the potent in vitro anti-SARS-CoV-2 efficacy that bovine lactoferrin has demonstrated with a more thorough examination, as well as to screen commercially available milk products for antiviral activities, which may be enhanced by the presence of other ingredients in addition to lactoferrin. To do this, the researchers designed an image-based SARS-CoV-2 inhibition assay using the human lung adenocarcinoma cell line H1437 which were chosen due to its expression of ACE2 and TMPRSS2, the 2 primary entry factors necessary for SARS-CoV-2 infection. The researchers tested bLF against some of the most common SARS-CoV-2 variants of concern. These included the WA1 variant (US 2020 strain), the B.1.1.7 variant (United Kingdom strain), B.1.351 variant (South Africa strain), P.1 variant (Japan/Brazil strain), and Delta variant (Indian strain). Notably, each of these variants includes modifications to the SARS-Cov-2 spike protein which endangers the efficacy of all newly produced vaccines. The results showed that bovine lactoferrin was effective against all the strains that were tested in vitro and the high specificity of bLF anti-SARS-CoV-2 activity, which is not observed for other bioactive milk proteins.
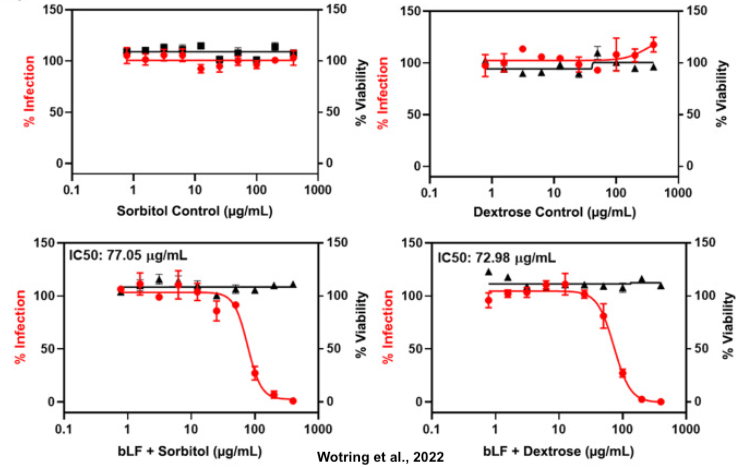
The study also sought to investigate formulation strategies for a chewable bLF tablet-placebo pair that retain efficacy in vitro. The team designed custom mixed berry flavored bLF tablet-placebo pairs formulated with either dextrose or sorbitol. They found that the excipients (dextrose and sorbitol) did not influence the potency of the sample which suggests that there is no formulation-dependent drop in efficacy and that these placebo-sample pairs would be a viable option for an anti-SARS-CoV-2 clinical trial.
References:- Wotring, J.W., Fursmidt, R., Ward, L. and Sexton, J.Z., 2022. Evaluating the in vitro efficacy of bovine lactoferrin products against SARS-CoV-2 variants of concern. Journal of Dairy Science.
- Mirabelli, C., Wotring, J.W., Zhang, C.J., McCarty, S.M., Fursmidt, R., Pretto, C.D., Qiao, Y., Zhang, Y., Frum, T., Kadambi, N.S. and Amin, A.T., 2021. Morphological cell profiling of SARS-CoV-2 infection identifies drug repurposing candidates for COVID-19. Proceedings of the National Academy of Sciences, 118(36).
- Wang, Y., Wang, P., Wang, H., Luo, Y., Wan, L., Jiang, M. and Chu, Y., 2020. Lactoferrin for the treatment of COVID‑19. Experimental and therapeutic medicine, 20(6), pp.1-1.
Other studies investigating lactoferrin against COVID-19
In September 2021, a research team in Italy reported the results of a survey based on real-life clinical practice, conducted by Italian general practitioners on their asymptomatic, paucisymptomatic, and moderate symptomatic COVID-19 patients, in home-based isolation, treated with bLf unloaded in liposome, alone or as supplementary treatment, depending on subject’s symptoms. The results showed the time required to achieve SARS-CoV-2 RNA negativization in Lf-treated patients (n = 82) was significantly lower (p < 0.001) compared to that observed in Lf-untreated ones (n = 39) (15 versus 24 days). Based on their data, the researchers concluded Lf could be an important supplementary treatment in counteracting SARS-CoV-2 infection, as it is also safe and well-tolerated by all treated patients.
In October 2021, a study reported a preliminary clinical trial on the efficacy of an oral and intranasal liposomal bLf formulation in asymptomatic and mild-to-moderate COVID-19 patients. A total of 92 mild-to-moderate (67/92) and asymptomatic (25/92) COVID-19 patients were recruited and divided into three groups. Thirty-two patients (14 hospitalized and 18 in home-based isolation) received only oral and intranasal liposomal bLf; 32 hospitalized patients were treated only with standard of care (SOC) treatment; and 28, in home-based isolation, did not take any medication. Liposomal bLf-treated COVID-19 patients obtained an earlier and significant (p < 0.0001) SARS-CoV-2 RNA negative conversion compared to the SOC-treated and untreated COVID-19 patients (14.25 vs. 27.13 vs. 32.61 days, respectively). Liposomal bLf-treated COVID-19 patients showed fast clinical symptom recovery compared to the SOC-treated COVID-19 patients. In bLf-treated patients, a significant decrease in serum ferritin, IL-6, and D-dimers levels was observed. No adverse events were reported.
Besides lactoferrin, ovotransferrin and lysozyme have been suggested by a study as potential antiviral and immune-modulating agents in COVID-19. The authors of the study think lactoferrin has direct antimicrobial effects through sequestering free iron and restoring iron homeostasis and also reduces oxidative stress and inflammation, which is pertinent to the COVID-19 pathology. Ovotransferrin shares many of the same activities as human/bovine lactoferrin and is more abundant than the latter. Peptides in ovotransferrin that have high sequence homology with these bovine lactoferrin and human lactoferrin peptides acting against herpes simplex virus, human cytomegalovirus and adenovirus, were shown to have double the antiviral activity compared with the bovine lactoferrin peptides. Lysozyme kills gram-positive bacteria through hydrolyzing the β-1,4 glycosidic bond between N-acetylglucosamine and N-acetylmuramic acid in the bacterial cell wall. It exerts antimicrobial effects through its cationic nature which enables it to bind to negatively charged surfaces (as in the case of lactoferrin), thereby expanding its activity well beyond that of gram-positive bacteria.
References:- Rosa, L., Tripepi, G., Naldi, E., Aimati, M., Santangeli, S., Venditto, F., Caldarelli, M. and Valenti, P., 2021. Ambulatory COVID-19 Patients Treated with Lactoferrin as a Supplementary Antiviral Agent: A Preliminary Study. Journal of clinical medicine, 10(18), p.4276.
- Campione, E., Lanna, C., Cosio, T., Rosa, L., Conte, M.P., Iacovelli, F., Romeo, A., Falconi, M., Del Vecchio, C., Franchin, E. and Lia, M.S., 2021. Lactoferrin as antiviral treatment in COVID-19 management: Preliminary evidence. International journal of environmental research and public health, 18(20), p.10985.
- Mann, J.K. and Ndung'u, T., 2020. The potential of lactoferrin, ovotransferrin and lysozyme as antiviral and immune-modulating agents in COVID-19. Future Virology, 15(9), pp.609-624.